TLDR;
This text explains hydrogen bonds, detailing their formation, characteristics, and impact on the properties of substances. It highlights the significance of electronegativity differences in creating partially positive hydrogen atoms and partially negative electronegative atoms, leading to strong dipole-dipole attractions. The text also provides examples of hydrogen bonding in everyday life, such as its role in the structure and stability of DNA.
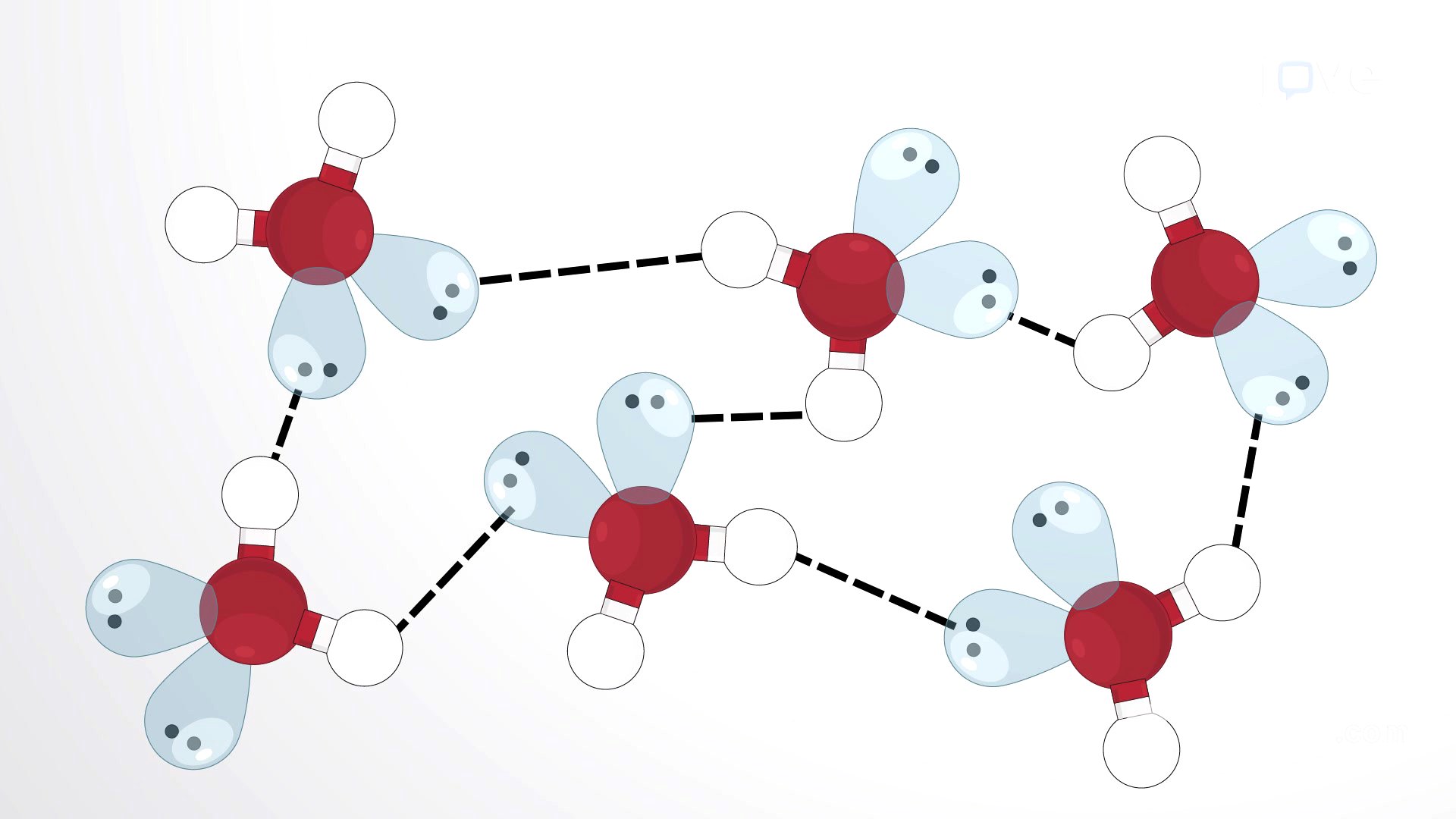
- Hydrogen bonds are formed between a partially positive hydrogen atom and another electronegative atom.
- Electronegativity differences between atoms like F, O, and N bonded to H create these bonds.
- Hydrogen bonds significantly affect the melting and boiling points of substances.
- They play a crucial role in the structure of DNA.
Hydrogen Bonds
A hydrogen bond occurs when a weakly positive hydrogen atom, already bonded to an electronegative atom, is attracted to another electronegative atom in a different polar molecule. Common examples include molecules like water, hydrogen fluoride, and ammonia. The significant electronegativity difference between the hydrogen atom and the atom it is bonded to, combined with the small size of the hydrogen atom, results in an unequal charge distribution. This unequal distribution makes the hydrogen atom partially positive and the electronegative atom partially negative. Consequently, molecules with F-H, O-H, or N-H groups are strongly attracted to similar groups in nearby molecules, forming a strong dipole-dipole attraction known as a hydrogen bond. Hydrogen bonds notably influence the properties of liquids and solids; for instance, methylamine has higher melting and boiling points than ethane, despite their similar size and mass, because methylamine's −NH group allows it to form hydrogen bonds, increasing intermolecular forces and raising these points. Hydrogen bonding is prevalent in nature, such as in DNA, where it forms base pairs through three hydrogen bonds, contributing to the shape and stability of the DNA double helix.