TLDR;
This video explores the fascinating history and science behind superglue, a powerful adhesive that has revolutionized various industries. It delves into the accidental discovery of cyanoacrylate, the chemical compound that makes up superglue, and explains how it works so quickly and effectively. The video also discusses the challenges of using superglue on certain materials, like plastics, and how it can be used underwater. It then explores the development of medical superglue, its applications in wound closure, and its potential to solve the plastic pollution problem.
- Superglue is a powerful adhesive that works by rapidly polymerizing, forming strong bonds between surfaces.
- It is highly reactive due to the electron-deficient nature of its molecules, which readily react with water and other negative ions.
- Superglue has various applications, including wound closure, but it also has limitations, such as its brittleness and inability to bond with certain plastics.
- Scientists are exploring the potential of superglue as a depolymerizable plastic, which could revolutionize plastic recycling and reduce environmental impact.
The Invention of Super Glue [0:00]
The video begins by introducing the concept of superglue's strength, showcasing its ability to hold significant weight with a single drop. It then delves into the accidental discovery of cyanoacrylate in 1942 by chemist Harry Coover while working on a clear plastic for gun sights. Coover initially dismissed the compound due to its tendency to stick to everything, but later rediscovered its potential as an adhesive in 1951 while working on jet plane canopies.
How Does Super Glue Work? [2:43]
This chapter explains the chemical process behind superglue's rapid bonding. Superglue is a liquid composed of identical monomer molecules of ethyl cyanoacrylate. When applied to a surface, these monomers react with each other, forming long polymer chains that solidify the glue. The presence of water, specifically the negative oxygen atoms and hydroxide ions, triggers this polymerization reaction, making superglue effective on various surfaces.
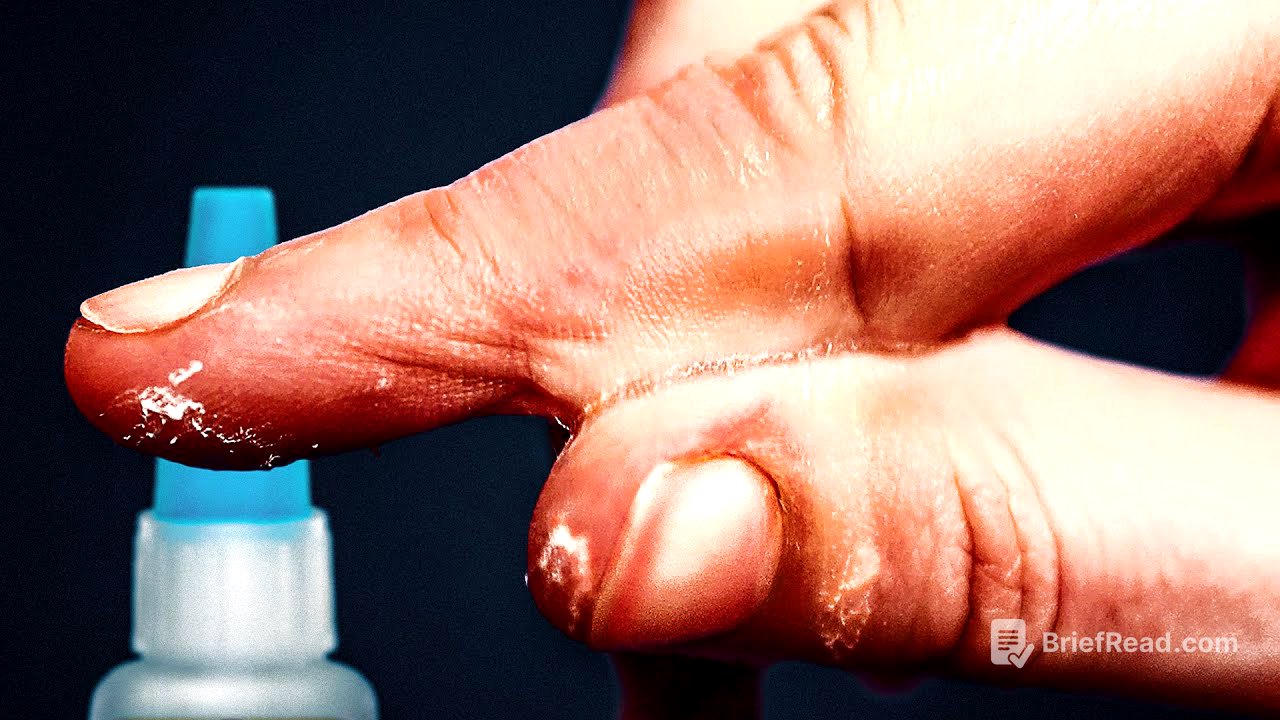
How To Get Superglue Off Your Skin [5:38]
The video highlights the challenges of removing superglue from skin due to its rapid polymerization and strong bonding with skin molecules. It emphasizes the importance of avoiding water and soap, as they can accelerate the reaction. Acetone, commonly found in nail polish remover, is presented as the most effective solution for removing superglue from skin. However, it warns against using acetone on eyes and emphasizes seeking medical attention in such cases.
Why Is Super Glue So Strong? [8:33]
This chapter delves into the strength of superglue bonds. The video explains that superglue polymers are primarily single chains running linearly between surfaces, creating a rigid structure similar to a hard hat. This rigidity makes superglue strong in compression and tension, allowing it to support significant weight. However, superglue is also brittle, meaning it can easily break under sudden impact or shear forces.
Why Doesn’t Super Glue Work on plastic? [12:00]
The video explores the limitations of superglue, specifically its inability to bond with certain plastics like polyethylene and polypropylene. These plastics are chemically inert, meaning they lack reactive sites for superglue's electron-deficient molecules to bond with. Their hydrophobic and non-porous nature further prevents superglue from adhering effectively.
Mixing Super Glue and Baking Soda [13:45]
This chapter discusses the effect of adding baking soda to superglue. Baking soda reacts with moisture in the air to produce hydroxide ions, which act as initiators for the polymerization reaction. This accelerates the setting process and creates a harder, more durable composite substance. The video demonstrates how baking soda can be used to strengthen joints and fill gaps, making the resulting material suitable for drilling and sanding.
Using Super Glue Underwater [15:30]
The video explores the possibility of using superglue underwater. It explains that gel cyanoacrylate, a type of superglue with thickeners, can be used underwater because the thickeners slow down the polymerization reaction, allowing for sufficient time to apply and detach objects. The video demonstrates the successful bonding of two objects underwater using gel cyanoacrylate.
Super Glue On Wounds [15:59]
This chapter focuses on the development of medical superglue. The video recounts the accidental discovery of superglue's potential for wound closure by Harry Coover's son. Coover recognized the potential and began researching medical applications, but faced challenges due to the heat generated during polymerization, the breakdown of superglue into toxic chemicals, and its brittleness. However, by increasing the number of carbons in the alkyl chain, Coover's team developed a modified superglue that addressed these issues, leading to the approval of medical superglue in 1998.
A Solution To Microplastics? [19:47]
The video concludes by exploring the potential of superglue as a solution to plastic pollution. It highlights the limitations of traditional plastic recycling methods, which degrade the quality of plastic and generate microplastics. Superglue, however, can be depolymerized back into its original monomers by heating it to 210 degrees Celsius. These monomers can then be distilled and reactivated, creating a sustainable and recyclable plastic. The video discusses the challenges of handling superglue's sticky nature and its brittleness, but presents solutions using inert plastics and a weak base to create longer, more stable polymer chains. The video concludes by emphasizing the importance of open-mindedness and curiosity in scientific exploration, highlighting Coover's ability to see beyond roadblocks and discover new possibilities.