TLDR;
Acids, bases, and neutralization reactions are crucial in biology, influencing the function of biological molecules and cellular signaling. Acids release hydrogen ions, while bases release hydroxyl ions or accept hydrogen ions. Neutralization reactions involve acids and bases, producing salt and water.
- Acids and bases are important in biology.
- Acids release hydrogen ions, while bases release hydroxyl ions or accept hydrogen ions.
- Neutralization reactions involve acids and bases, producing salt and water.
Acids, Bases and Neutralization Reactions
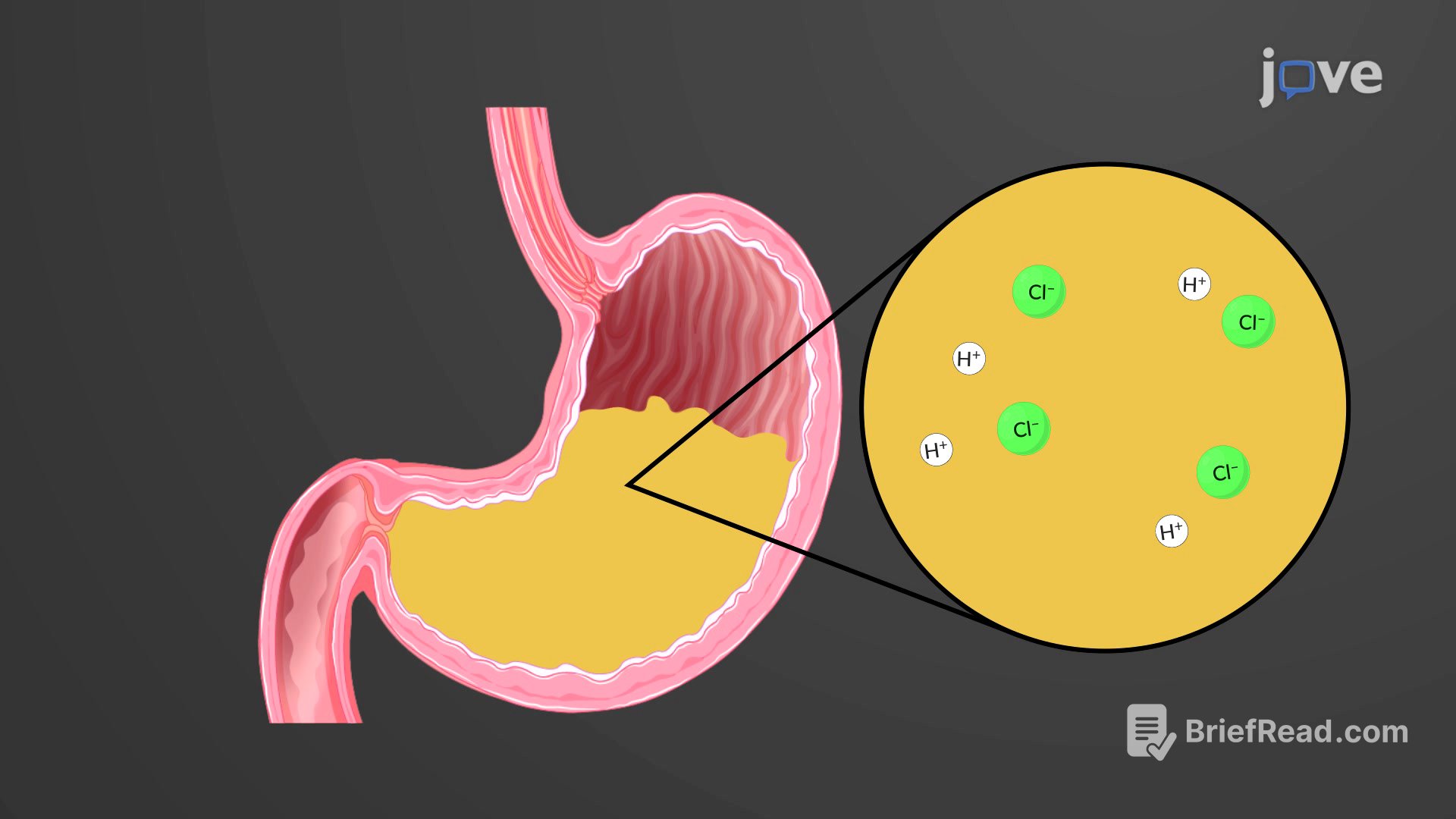
Acids and bases are very important in biology because the pH of a biological system can significantly affect the function of biological molecules, including enzymes, proteins, and nucleic acids. Acids release hydrogen ions in solution, with strong acids ionizing completely and weak acids not fully ionizing. Bases release hydroxyl ions or accept hydrogen ions, reducing acidity. Neutralization reactions occur when an acid and base react to form salt and water, playing a central role in various natural and technological processes.
![[Digimon Podcast] LiT Episode 83 - I choo, choo, choose you](https://wm-img.halpindev.com/p-briefread_c-10_b-10/urlb/aHR0cDovL2ltZy55b3V0dWJlLmNvbS92aS91WE52RXpJc3JfQS9ocWRlZmF1bHQuanBn.jpg)







