TLDR;
This video provides an overview of conformational analysis, focusing on the differences between configurations and conformations, energy profile diagrams, and types of strain in molecules. It also discusses the structures of cyclooctane and cyclodecane, including their preferred conformations and the concept of transannular strain.
- Configurations vs. Conformations
- Energy Profile Diagrams
- Types of Strain: Baeyer Strain, van der Waals Strain, Torsional Strain, Transannular Strain
- Structures of Cyclooctane and Cyclodecane
Conformational Analysis: Configurations vs. Conformations [0:00]
The discussion begins by differentiating between configurations and conformations. Configurations are distinct arrangements of atoms that can only be interconverted by breaking and forming bonds, while conformations are different arrangements that can be interconverted through simple bond rotations. The stability of conformations is studied using energy profile diagrams, which plot energy against the dihedral angle. For ethane, the staggered conformation is more stable than the eclipsed conformation due to reduced repulsion between hydrogen atoms. Conformations are numerous, representing all possible arrangements, whereas conformers are specific conformations corresponding to the minima on the energy profile diagram.
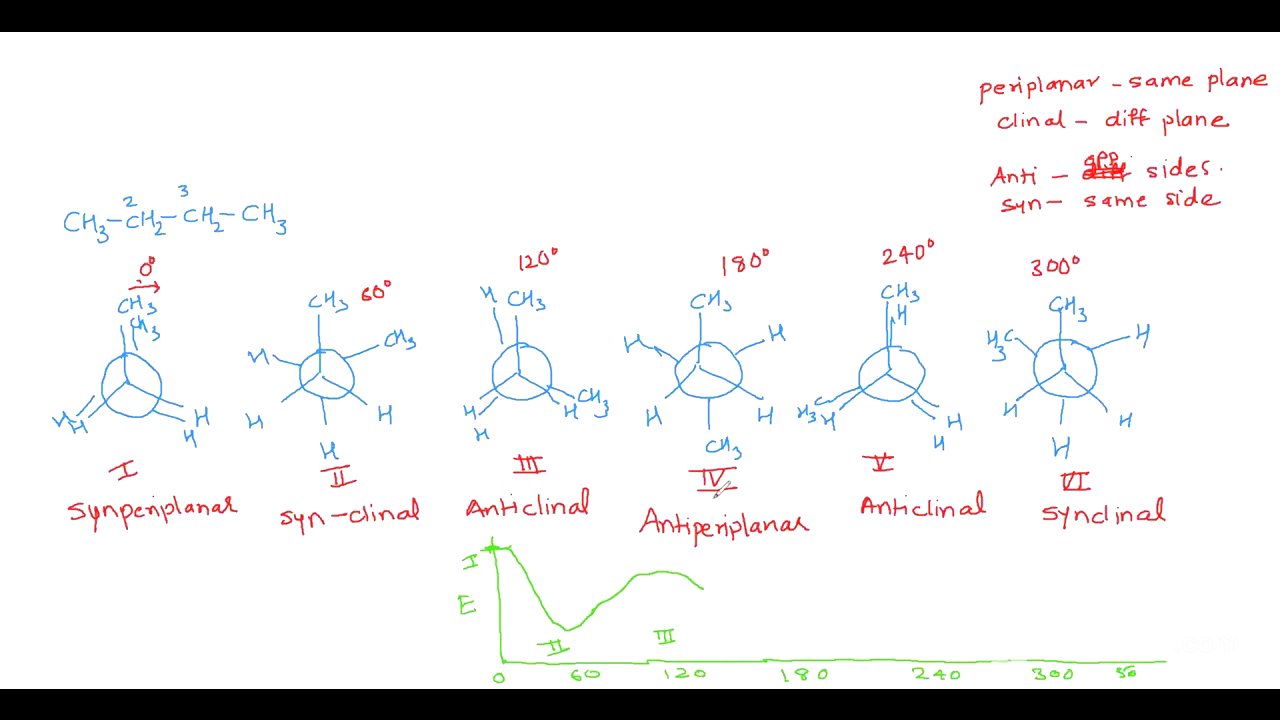
Butane Conformations and Nomenclature [5:41]
The video explains the conformations of butane, focusing on the dihedral angle between the two methyl groups. It introduces the terms periplanar (groups in the same plane) and clinal (groups in different planes), as well as anti (groups on opposite sides) and syn (groups on the same side), to describe different conformations. The conformations are named based on these terms, such as syn-periplanar, synclinal, anticlinal, and anti-periplanar. The energy profile diagram for butane shows three minima, corresponding to three conformers, with the anti-periplanar conformer being the most stable, also known as the anti-butane unit.
Types of Strain: Baeyer, Steric, Torsional, and Transannular [12:06]
The video describes four types of strain relevant to conformational analysis. Baeyer or angle strain arises from the deviation of bond angles from their ideal values, calculated using a formula involving the reference angle and the internal angle of the cyclic chain. van der Waals or steric strain results from the repulsion between atoms that come closer than the sum of their van der Waals radii. Torsional strain is due to the resistance to free rotation around a bond, which is associated with an energy barrier. Transannular strain occurs in medium-sized rings when atoms across the ring interact, causing strain.
Cyclooctane and Cyclodecane Structures [18:53]
The discussion shifts to the structures of cyclooctane and cyclodecane. Cyclooctane prefers a boat-chair conformation over the crown form, with transannular strain occurring between hydrogen atoms on carbons 1, 4, and 6, as well as between carbons 3, 5, and 7. These hydrogens involved in the strain are called intra-annular hydrogens, while others are peripheral hydrogens. Cyclodecane adopts a boat-chair-boat conformation, with transannular interactions between hydrogens on carbons 1, 4, and 8, and between carbons 3, 6, and 9. The ten carbons in cyclodecane are divided into three types based on whether the axial or equatorial proton is intra-annular or peripheral. The structure of cyclodecane resembles that of diamond lattices. The video concludes by mentioning that replacing carbon atoms with heteroatoms or changing the hybridization from sp3 to sp2 can reduce strain in these rings. Geminal substitution can also restrict ring flipping, maintaining a rigid conformation.