TLDR;
This video provides an overview of pharmacogenomics, explaining how genetic variations influence drug responses. It covers the ADME process, the role of enzymes like cytochrome P450, and the differences between fast and slow metabolizers. The video also presents real-world examples in oncology, pain management, mental health, cardiology, neurology, and infectious diseases, highlighting how genetic testing can improve treatment outcomes. Additionally, it explores future directions, including the use of AI, the role of RNA and epigenetics, and the impact of the microbiome on personalized medicine.
- Pharmacogenomics tailors medications to individual genetic profiles, optimizing effectiveness and reducing side effects.
- Genetic variations affect drug metabolism, efficacy, and safety, influencing treatment outcomes.
- Emerging technologies like AI and microbiome research are driving advancements in personalized medicine.
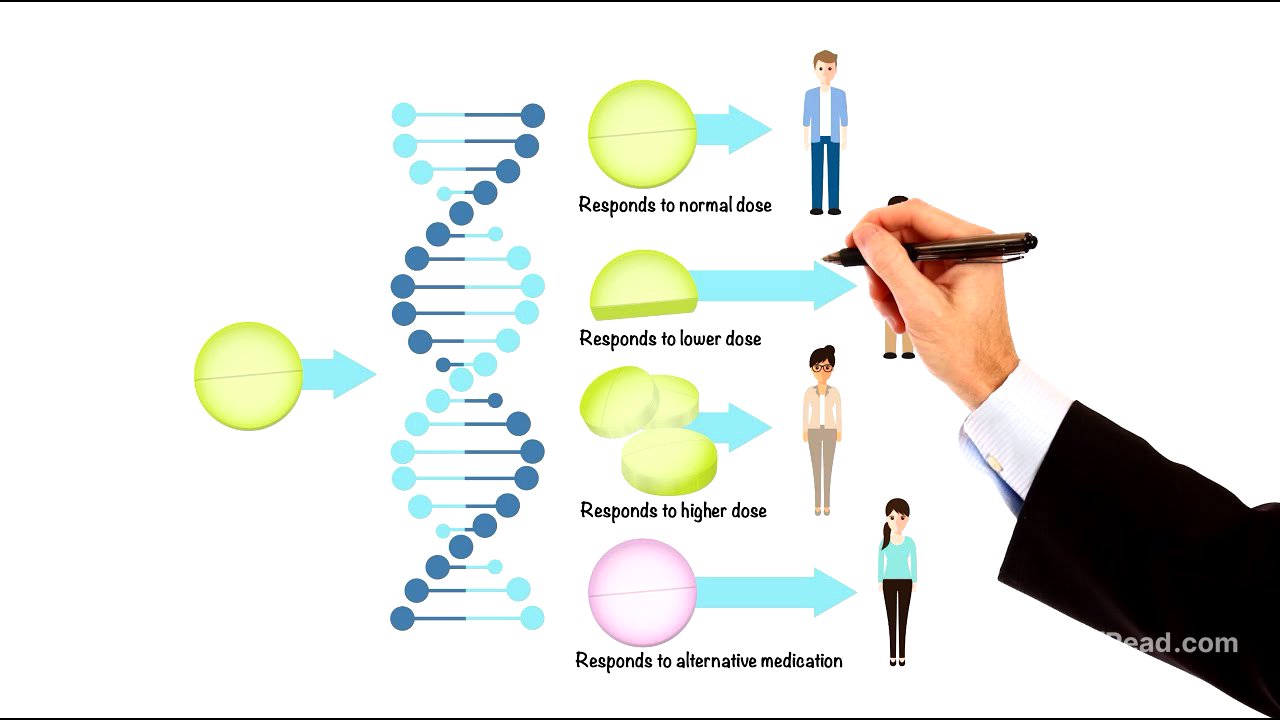
What is Pharmacogenomics? [0:00]
Pharmacogenomics is a field that combines genetics and pharmacology to tailor medications to an individual's unique genetic profile. This approach aims to reduce side effects and optimize the effectiveness of treatments. By understanding how genetic variations influence drug responses, pharmacogenomics paves the way for precision medicine, where treatments are customized to each patient's biology.
The ADME Process [1:03]
The ADME process describes how medications work in the body, involving absorption, distribution, metabolism, and elimination. Metabolism, primarily handled by cytochrome P450 (CYP) enzymes in the liver, is crucial because these enzymes break down drugs for effective use or elimination. Genetic variations can affect the speed at which these enzymes work, leading to differences in drug metabolism among individuals.
Fast vs. Slow Metabolizers [2:03]
Individuals can be classified as fast or slow metabolizers based on the activity of their enzymes. Fast metabolizers break down drugs quickly, potentially reducing the drug's effectiveness, while slow metabolizers may experience a buildup of the drug, increasing the risk of side effects or toxicity. These differences are often due to genetic variations in the genes that code for these enzymes and transport proteins like P-glycoprotein, which affect how drugs move in and out of cells. Even receptors, the proteins that drugs target, can differ genetically, influencing the body's response to medication.
Oncology [3:30]
In oncology, pharmacogenomics allows for tailoring cancer treatments based on a patient's genetic profile and the specific mutations in their tumor. For example, in HER2-positive breast cancer, genetic testing identifies patients who are good candidates for targeted therapies like trastuzumab, which blocks HER2 receptors and slows cancer progression. Similarly, imatinib is prescribed for chronic myeloid leukemia (CML) based on the presence of the BCR-ABL fusion gene, increasing the likelihood of successful treatment while minimizing harm to healthy cells. Imatinib competitively binds to the ATP binding site of BCR-ABL, preventing phosphorylation of substrate proteins and shutting down abnormal signaling cascade.
Pain Management [5:33]
Pharmacogenomics plays a role in pain management, particularly with drugs like codeine. The CYP2D6 enzyme converts codeine into its active form, morphine. Genetic variations in the CYP2D6 gene can cause individuals to be fast or slow metabolizers. Fast metabolizers may experience side effects like excessive drowsiness, while slow metabolizers may not get any pain relief. Knowing a patient's CYP2D6 status helps doctors choose a more effective and safer alternative.
Mental Health [6:12]
In mental health, pharmacogenomics is used to optimize antidepressant treatments. Variations in the SLC6A4 gene, which encodes the serotonin transporter, affect how well selective serotonin reuptake inhibitors (SSRIs) work. The 5-HTTLPR polymorphism in this gene has short (S) and long (L) alleles, impacting serotonin transporter expression and serotonin availability. Individuals with the SS variant have lower transporter expression and may respond less effectively to SSRIs, while those with the LL variant have higher transporter expression and typically respond better to SSRIs. Similarly, the CYP2C19 gene, which metabolizes SSRIs like citalopram, can guide personalized treatment choices based on a patient's genetic profile.
Cardiology [7:51]
Pharmacogenomics is making strides in cardiology, particularly with drugs like clopidogrel, a blood thinner. The effectiveness of clopidogrel depends on the CYP2C19 enzyme to activate the drug. Some patients have variations in this enzyme that render the drug ineffective, putting them at risk for blood clots. Genetic testing can identify these patients, allowing doctors to prescribe alternative treatments like ticagrelor or prasugrel.
Neurology [8:28]
In neurology, pharmacogenomics has proven invaluable in treating epilepsy. Carbamazepine, a drug used to manage seizures, carries a risk of severe skin reactions like Stevens-Johnson syndrome in patients with a specific genetic variant in the HLA gene, particularly HLA-B*1502. Genetic screening can identify at-risk individuals, preventing these potentially life-threatening side effects.
Infectious Diseases [9:00]
Pharmacogenomics influences the field of infectious diseases. Abacavir, an anti-retroviral drug used to treat HIV, can cause severe hypersensitivity reactions in individuals with the HLA-B*5701 gene variant. Genetic testing ensures that only patients without this variant are prescribed the drug, improving safety and adherence.
AI & Big Data [9:49]
As pharmacogenomics evolves, emerging technologies like artificial intelligence (AI) are paving the way for more precise medicine. AI enables the analysis of vast and complex genetic data sets, predicting patient responses to medications, identifying those at risk of adverse drug reactions, and accelerating drug discovery by suggesting new targets based on genetic markers. AI integrates clinical, environmental, and genetic data to provide a comprehensive understanding of drug efficacy and safety.
RNA in Pharmacogenomics [11:00]
RNA and epigenetic changes add extra layers of regulation that influence how individuals respond to medications. RNA translates DNA's instructions into proteins, affecting how drugs interact with the body. Non-coding RNAs, such as microRNAs, regulate gene expression by binding to mRNA and inhibiting translation or promoting mRNA degradation. Researchers are studying these RNA markers to predict patient responses to treatments, particularly in cancer.
Epigenetics [12:00]
Epigenetics involves modifications that affect gene expression without altering DNA. DNA methylation can silence genes involved in drug metabolism, while histone modifications can alter how accessible genes are for transcription. These epigenetic factors add another layer of complexity to understanding individual drug responses.
Pharmacomicrobiomics [12:28]
Pharmacomicrobiomics explores the interplay between an individual's genetic makeup and their microbiome. Gut microbes produce enzymes that can activate, deactivate, or modify drugs in the human body. Variations in host genes affect which microbes thrive in the gut, creating a dynamic interplay between human genetics and microbiome composition. Researchers are exploring strategies like probiotics and fecal microbiota transplants to optimize drug responses.
Conclusion - Personalized Medicine [14:15]
Pharmacogenomics is paving the way for personalized medicine by understanding the roles of genetics, epigenetics, RNA, and the microbiome. Cutting-edge technologies like AI and microbiome research are driving advancements toward more effective, safer, and tailored treatments. This transformative field aims to create a future where healthcare is precise, proactive, and uniquely designed for each individual.