TLDR;
This video by the Amoeba Sisters explains the importance of ATP (adenosine triphosphate) in cells. It clarifies that ATP is not just a generic energy currency but a nucleotide derivative crucial for various cellular processes. The video covers ATP's structure, how cells generate ATP through processes like cellular respiration and fermentation, and how ATP works by coupling its hydrolysis to endergonic reactions, often through phosphorylation.
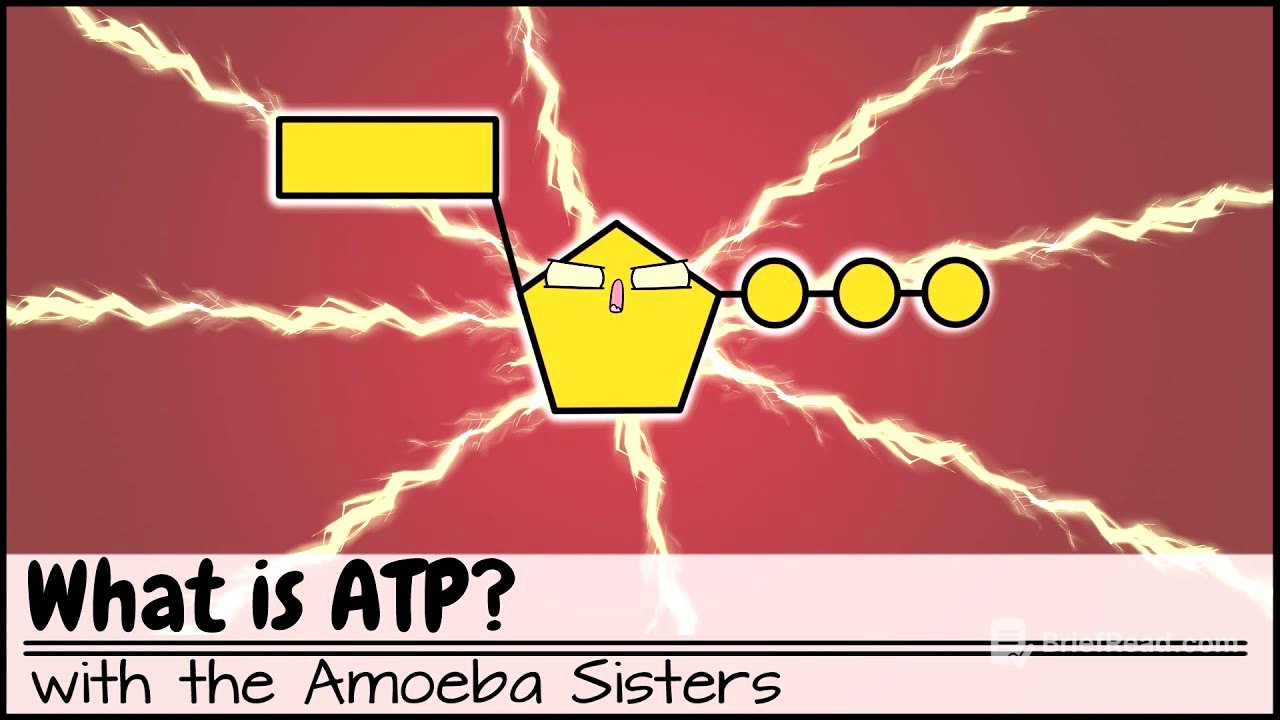
- ATP is a nucleotide derivative composed of adenine, ribose, and three phosphate groups.
- Cells generate ATP through aerobic and anaerobic respiration, as well as fermentation.
- ATP hydrolysis releases energy that is coupled to endergonic processes via phosphorylation.
Some Examples of ATP Uses in Cell Processes [0:40]
ATP is essential for numerous cellular functions, including active transport, where cells move substances against their concentration gradients. It also plays a critical role in muscle contraction through the interaction of actin and myosin. Additionally, ATP is vital for cell signaling, enabling cells to communicate effectively.
What is ATP? [1:14]
ATP is a nucleotide derivative, similar to DNA and RNA, comprising a nitrogenous base (adenine), a sugar (ribose), and three phosphate groups. Its full name, adenosine triphosphate, indicates the presence of adenine and three phosphates. The arrangement of these components gives ATP its unique properties.
How do we get ATP? [1:52]
All cells require ATP and produce it through various processes. These processes can be either aerobic, like cellular respiration (which uses oxygen), or anaerobic, like fermentation (which doesn't use oxygen). Plants generate ATP by breaking down glucose produced during photosynthesis, while animals break down consumed glucose. This ATP production is part of a cycle where ATP is hydrolyzed to release energy and a phosphate, becoming ADP, and then regenerated back into ATP through processes like cellular respiration.
How does ATP work? [3:05]
ATP's functionality extends beyond simply releasing energy upon hydrolysis. The bond between the second and third phosphate groups contributes to ATP's instability. When ATP is hydrolyzed, it becomes more stable ADP, releasing free energy in an exergonic reaction. This energy release is coupled to endergonic processes through phosphorylation, where a phosphate group is transferred to another molecule, making it more reactive. For example, in active transport, a transport protein is phosphorylated, enabling it to move molecules against their concentration gradient.