TLDR;
This text introduces chemical reactions, their components, and the factors influencing them. It covers reactants, products, the law of conservation of mass, energy requirements like activation energy, and the impact of concentration and temperature. The text also explains chemical equations, reversible reactions, equilibrium, and how these principles apply to biological systems, particularly in maintaining homeostasis in blood.
- Chemical reactions involve reactants forming products while conserving mass.
- Activation energy, concentration, and temperature affect reaction rates.
- Reversible reactions can reach equilibrium, crucial in biological systems like blood homeostasis.
Introduction to Chemical Reactions
Chemical reactions start with reactants, which are substances that enter the reaction. For instance, sodium and chloride ions are reactants in creating table salt. The substances produced by a chemical reaction are called products. Chemical reactions adhere to the law of conservation of mass, meaning matter is neither created nor destroyed. The elements and number of atoms in the reactants are fully present in the product, and vice versa.
Energy in Chemical Reactions
Chemical reactions need sufficient energy for matter to collide with enough force to break old chemical bonds and form new ones. Kinetic energy powers matter in motion, while potential energy is based on position or structure. Atoms always have kinetic energy due to their constant motion. Activation energy is required to break the chemical bonds of reactants and initiate a reaction. The rate of a chemical reaction can be influenced by the concentration and temperature of the reactants.
Chemical Equations and Reversible Reactions
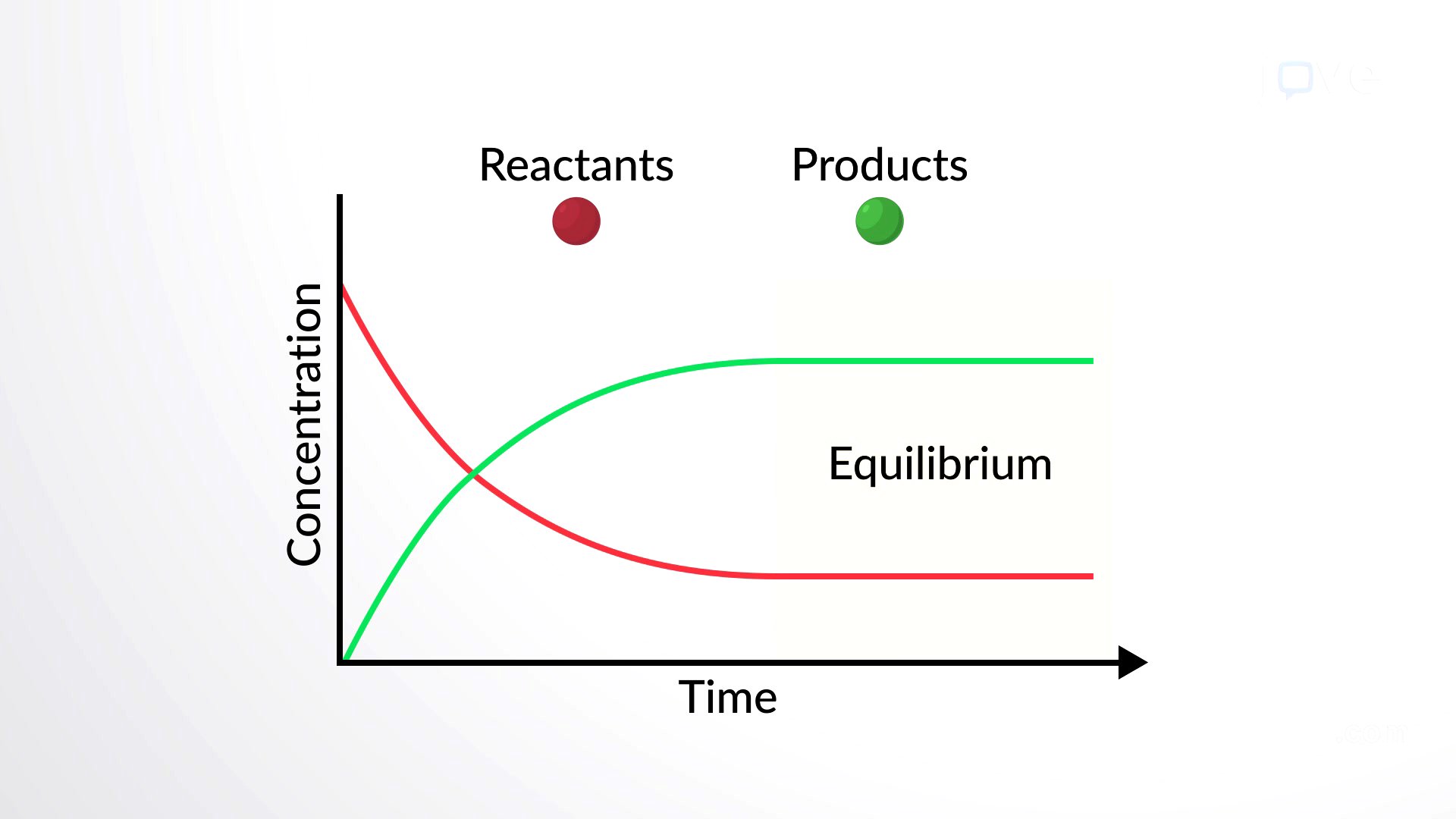
Chemical equations are written with reactant formulas on the left and product formulas on the right of a reaction arrow, indicating the reaction's direction. Reversible reactions can proceed in both directions. Equilibrium is reached when the rates of forward and reverse reactions are equal, maintaining constant concentrations of reactants and products. Equilibrium systems can vary, containing mostly products, mostly reactants, or a mix of both.
Equilibrium in Biological Systems
In human blood, excess hydrogen ions bind to bicarbonate ions, forming an equilibrium with carbonic acid. Adding carbonic acid would cause some conversion back to bicarbonate and hydrogen ions. Biological reactions rarely reach equilibrium due to constantly changing concentrations of reactants or products. For example, carbonic acid formed from excess hydrogen ions can leave the body as carbon dioxide, driving the reaction forward and maintaining blood homeostasis.