TLDR;
This text introduces the concept of chemical bonds, which are essential for the formation of molecules and compounds. It explains how atoms achieve stability by forming ionic, covalent, and hydrogen bonds.
- Atoms form chemical bonds to achieve a stable electron configuration, typically by satisfying the octet rule.
- Ionic bonds involve the transfer of electrons between atoms, creating positively charged cations and negatively charged anions that attract each other.
- Covalent bonds involve the sharing of electrons between atoms and are stronger and more common in organic molecules.
- Hydrogen bonds are weak attractions between a weakly positive hydrogen atom in one molecule and an electronegative atom in another molecule, crucial for water's properties.
Introduction to Chemical Bonds
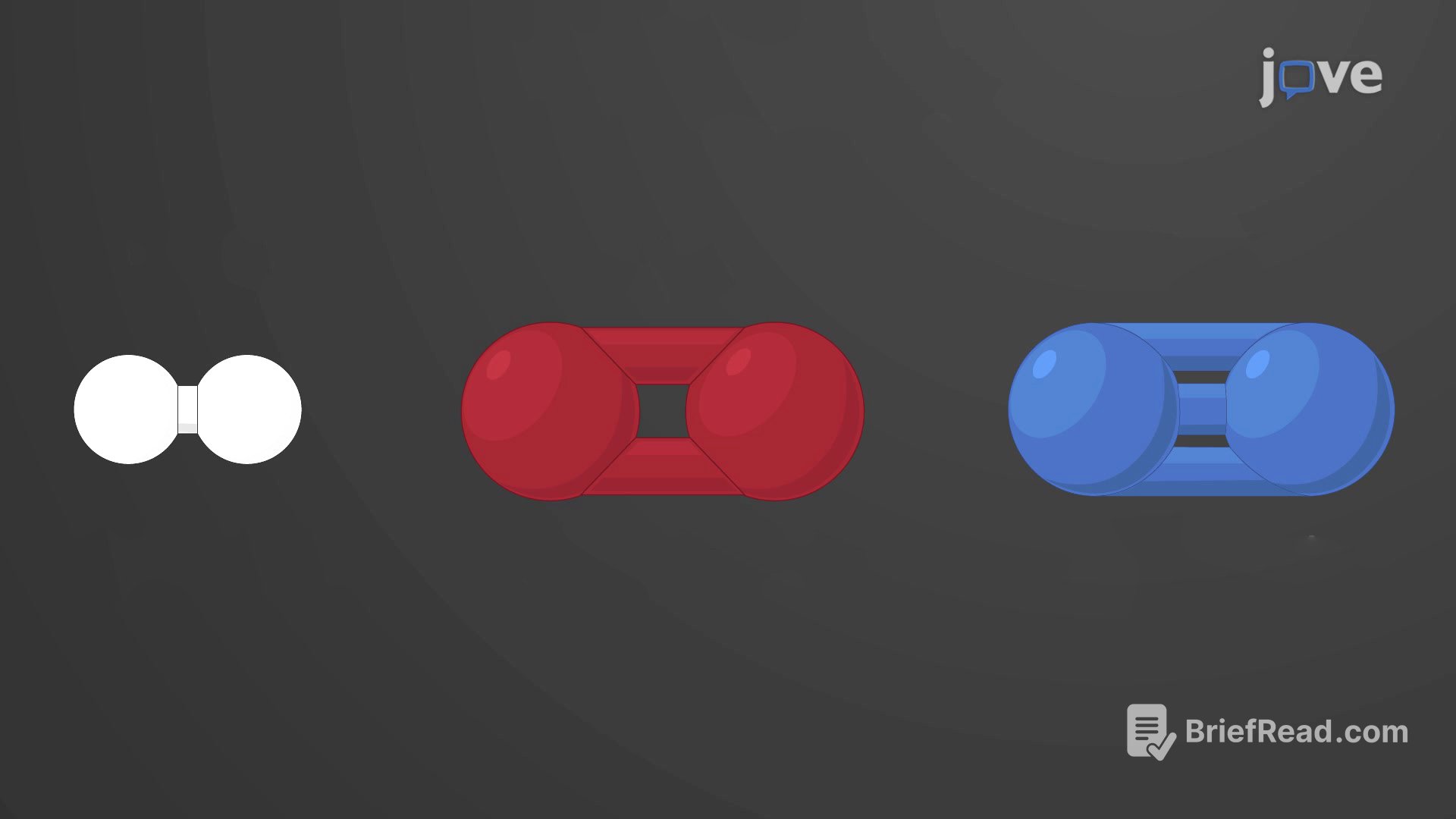
The stability of an atom and its ability to form chemical bonds are determined by the electrons in its outermost energy level. Atoms strive to have eight electrons in their valence shell (octet rule), except for the innermost shell, which can hold a maximum of two electrons. When atoms lack enough electrons to fill their outermost shells, they form chemical bonds with other atoms to achieve a stable electronic configuration. A chemical bond is the force that holds atoms together in a molecule or compound.
Ionic Bonds
Atoms can gain or lose electrons to fill their valence shell, becoming electrically charged ions. A cation is a positively charged ion formed by losing electrons, while an anion is a negatively charged ion formed by gaining electrons. The opposite charges of cations and anions create a mutual attraction, forming an ionic bond, which is a close association between ions of opposite charge. Common table salt is an example of a compound formed through ionic bonding.
Covalent Bonds
Covalent bonds are formed through the sharing of electrons between atoms, satisfying the octet rule. These bonds are stronger and more prevalent than ionic bonds in living organisms' molecules. Covalent bonds are commonly found in carbon-based organic molecules like DNA and proteins, as well as in inorganic molecules such as water, carbon dioxide, and oxygen.
Hydrogen Bonds
A hydrogen bond occurs when a weakly positive hydrogen atom, already bonded to an electronegative atom, is attracted to another electronegative atom from a different molecule. A common example is the hydrogen bonding between water molecules, where the weakly negative oxygen atom in one water molecule attracts the weakly positive hydrogen atoms of other water molecules. This phenomenon is observed when raindrops merge or when a creek flows into a river.