TLDR;
This text explains covalent bonds, focusing on how they form through electron sharing between atoms and how electronegativity influences the polarity of these bonds. It highlights the differences between polar and nonpolar molecules and their interactions with water, which is crucial for cellular processes.
- Covalent bonds form when atoms share electrons to achieve stable valence shells.
- Electronegativity determines whether a covalent bond is polar or nonpolar.
- Polar molecules are hydrophilic and dissolve in water, while nonpolar molecules are hydrophobic and do not dissolve in water.
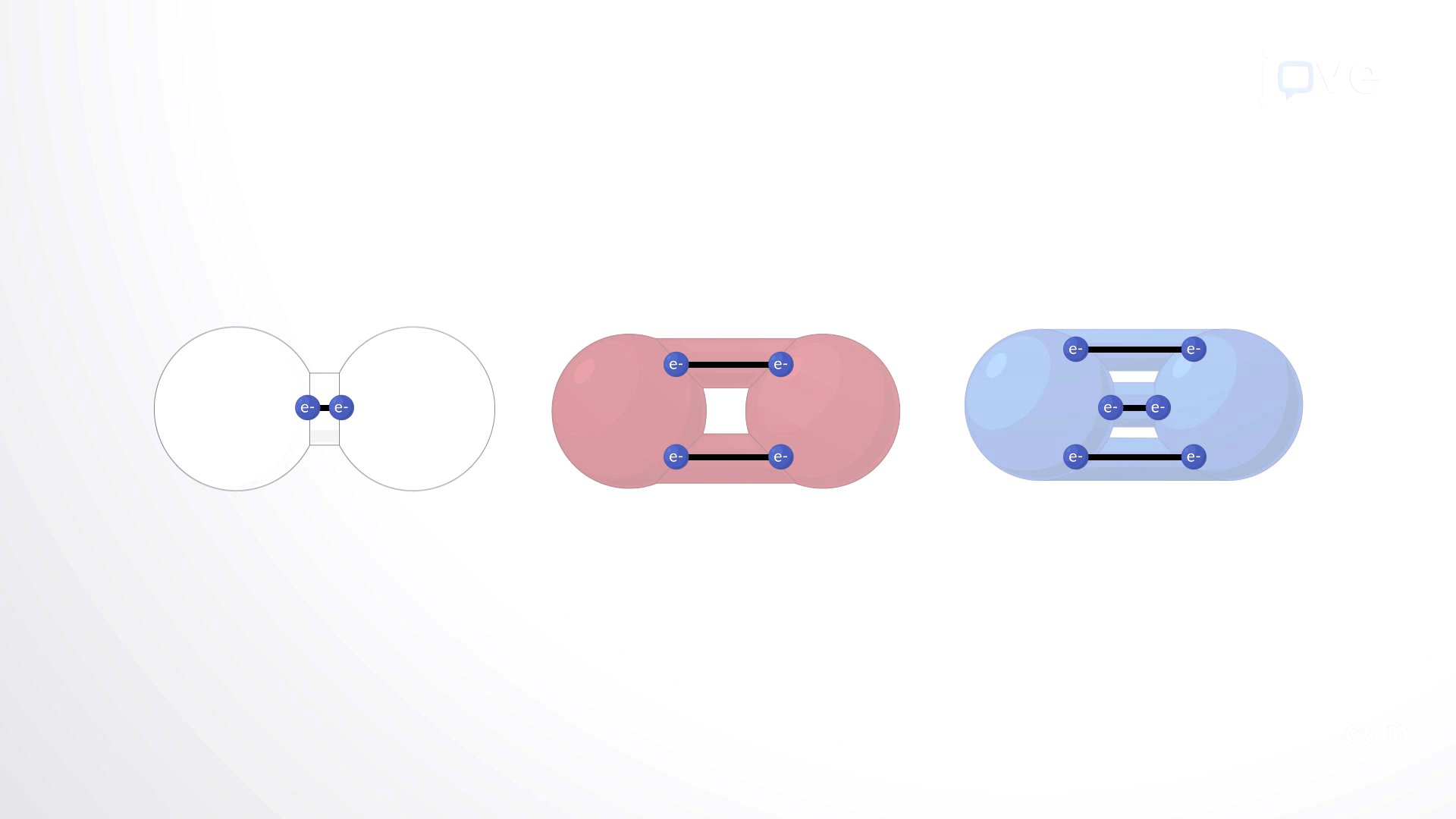
Covalent Bonds Overview
Covalent bonds are created when atoms share electrons to complete their valence shells. The electronegativity of an atom, which is the measure of its ability to attract shared electrons, determines how these electrons are shared. If atoms have similar electronegativities, they form nonpolar covalent bonds where electrons are shared equally. Conversely, if atoms have different electronegativities, they form polar bonds where electrons are shared unequally. The number of covalent bonds an atom can form depends on its number of valence electrons; for example, oxygen, with six valence electrons, can form single bonds with two other atoms or a double bond with one other atom. Carbon, having four valence electrons, can form four covalent bonds. When a covalent bond forms, the atoms share a pair of electrons in a hybrid orbital, altering the electron's path around both nuclei. Covalent bonds are strong and resistant to physical forces once established.
Electronegativity Determines Whether a Molecule Is Polar or Nonpolar
Electronegativity is an atom's tendency to attract electrons within a bond. Fluorine is the most electronegative element, and electronegativity generally decreases down and to the left on the periodic table. Significant differences in electronegativity between atoms typically lead to the formation of ions rather than covalent bonds. For atoms forming covalent bonds, electronegativity determines bond polarity. Nonpolar bonds involve equal electron sharing, resulting in no charge across the molecule, while polar bonds occur when one atom is more electronegative, creating partial negative and positive charges. Polar molecules are hydrophilic due to their partial charges, making them soluble in water, whereas nonpolar molecules, like those containing long hydrocarbon chains, are hydrophobic and do not dissolve in water. The interaction of molecules with water and other charged molecules affects their transport and utilization within cells, which are surrounded by fluid and contain water-based cytoplasm.