TLDR;
This text defines key atomic concepts: atomic number, mass number, and isotopes. The atomic number defines an element by its number of protons, while the mass number is the sum of protons and neutrons. Isotopes are atoms of the same element with different numbers of neutrons, leading to variations in mass.
- Atomic number defines the element.
- Mass number is the sum of protons and neutrons.
- Isotopes are atoms of the same element with different numbers of neutrons.
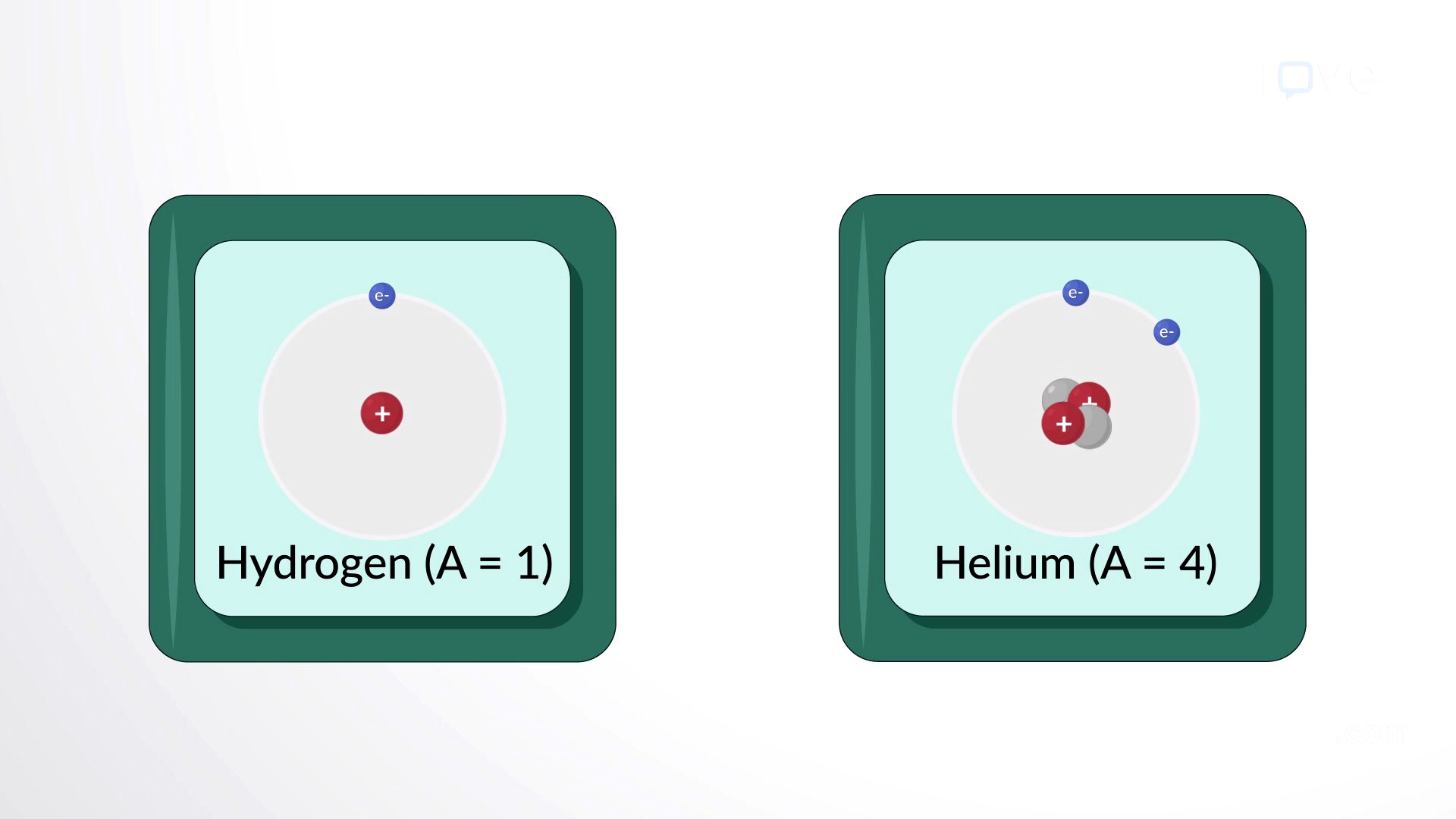
Atomic Number and Mass Number
The atomic number of an atom is determined by the number of protons in its nucleus, which defines the element. In a neutral atom, the number of protons equals the number of electrons. The mass number is the total number of protons and neutrons in an atom's nucleus. The number of neutrons can be found by subtracting the atomic number from the mass number.
Isotopes
Isotopes are atoms of the same element that have different masses due to varying numbers of neutrons. While isotopes have different masses, they are chemically identical because they possess the same number of protons. Frederick Soddy's discovery of isotopes earned him the Nobel Prize in Chemistry in 1921.