TLDR;
This video explains the stereochemistry of six-membered rings, focusing on cyclohexane. It details the stability of cyclohexane in its chair form due to the tetrahedral arrangement of carbon bonds, which eliminates angle strain. The video further describes axial and equatorial hydrogen positioning, the transformation of chair form to boat form, and the strains associated with the boat form, including flagpole interactions and torsional strain.
- Cyclohexane's chair form is more stable due to the absence of angle strain.
- Axial and equatorial hydrogens alternate positions on the cyclohexane ring.
- The boat form of cyclohexane has higher potential energy due to bond opposition strain and flagpole interactions.
Introduction to Six-Membered Rings and Cyclohexane [0:05]
Six-membered rings, like cyclohexane, are more stable than five-membered rings. Cyclohexane, in its non-planar structure, exhibits tetrahedral angles (109° 28') between all carbon valence bonds, making it free of angle strain. The chair form of cyclohexane is particularly stable because each carbon atom is attached with hydrogen at a tetrahedral angle, ensuring minimal strain.
Axial and Equatorial Hydrogens in Cyclohexane [1:09]
In the chair form of cyclohexane, hydrogen atoms are positioned either axially (ha) or equatorially (he). Axial hydrogens are oriented alternately above and below the ring, while equatorial hydrogens also alternate in a similar manner. This arrangement can be visualized using Newman projections, which show the staggered arrangement of hydrogens, minimizing interaction and strain.
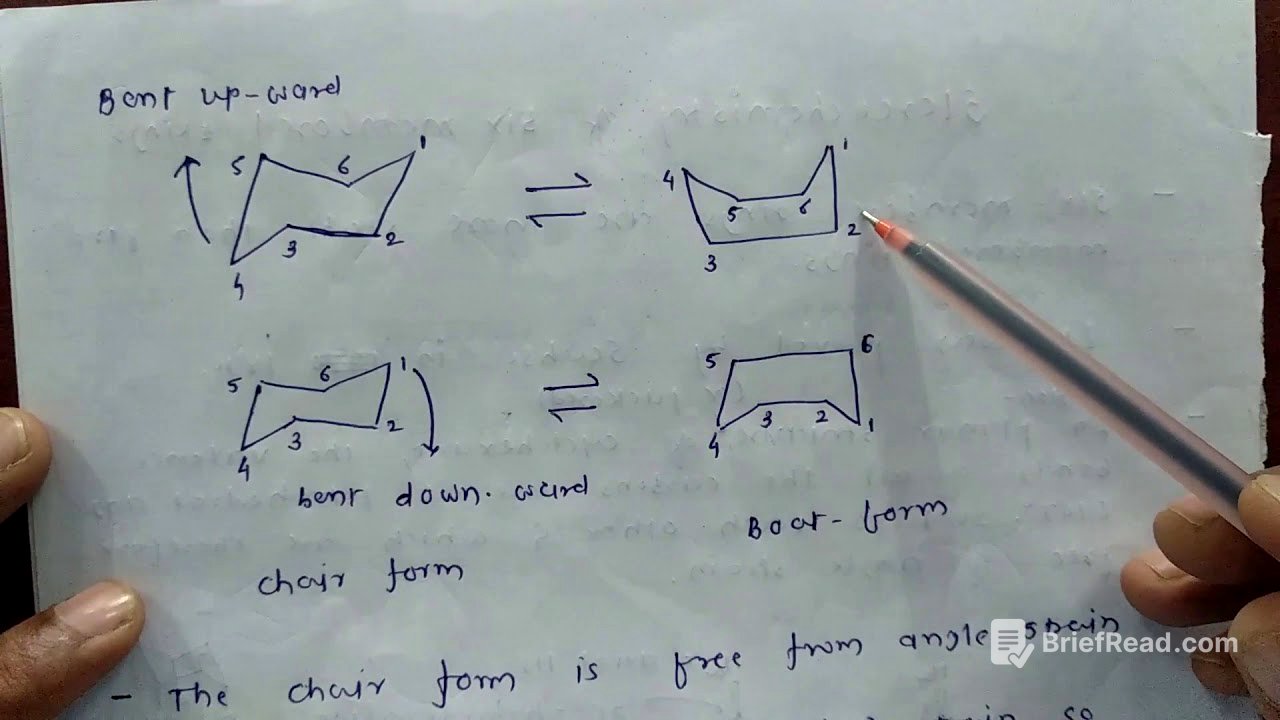
Chair Form Mobility and Transformation to Boat Form [3:37]
The chair form of cyclohexane is mobile and can transform into various shapes, including the boat form. This transformation occurs when the bottom part of the chair is bent upwards or downwards. While the boat form is free from angle strain, it has higher potential energy due to bond opposition strain.
Bond Opposition Strain and Flagpole Interactions in Boat Form [5:31]
The boat form of cyclohexane experiences bond opposition strain due to the eclipsing of hydrogen atoms. Specifically, the flagpole hydrogens, which are 1.8 angstroms apart, create significant repulsion because this distance is less than the sum of their van der Waals radii (2.4 angstroms). This interaction, known as flagpole interaction, increases the molecule's energy and reduces its stability. The eclipsing of carbon-carbon bonds also contributes to torsional strain, further destabilizing the boat conformation.
![Miris! Intoleransi dalam Beragama Masih Terjadi di Indonesia [Metro Siang]](https://wm-img.halpindev.com/p-briefread_c-10_b-10/urlb/aHR0cDovL2ltZy55b3V0dWJlLmNvbS92aS9UZDV4SDF3X2gzdy9ocWRlZmF1bHQuanBn.jpg)