TLDR;
This video provides a recap on writing electron configurations and serves as preparation for understanding quantum numbers. It explains electron configuration as the arrangement of electrons in energy levels around an atomic nucleus, represented by numbers, letters, and superscripts. The video details the three key principles for writing electron configurations: the Aufbau principle, Pauli's exclusion principle, and Hund's rule. It also covers how to determine the atomic number of an element using the periodic table and how electron configurations relate to magnetic properties. The video concludes with examples of writing electron configurations for phosphorus and oxygen.
- Electron configuration is the arrangement of electrons in energy levels.
- Three principles guide electron configuration: Aufbau, Pauli's exclusion, and Hund's rule.
- Electron configurations determine magnetic properties of elements.
Introduction to Electron Configuration [0:00]
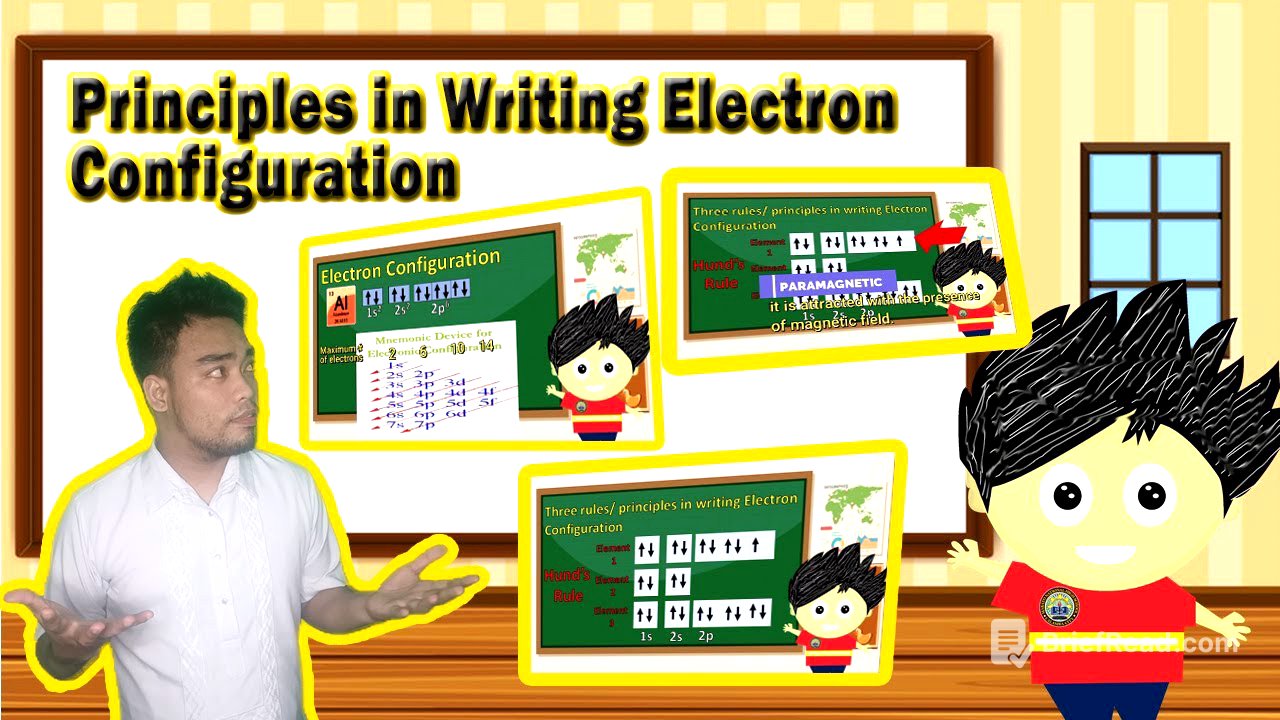
The video introduces electron configuration as the arrangement of electrons in the energy levels around an atom's nucleus. It is a shorthand representation of how electrons are arranged within orbitals, levels, and sublevels. The representation includes numbers indicating the principal quantum number or energy level, letters representing orbitals or sublevels (s, p, d, f), and superscripts indicating the number of electrons in each sublevel.
Understanding Orbitals and Sublevels [1:08]
The different sublevels have varying capacities for accommodating electrons. The s sublevel can hold a maximum of 2 electrons, the p sublevel can hold up to 6 electrons, the d sublevel can hold up to 10 electrons, and the f sublevel can hold a maximum of 14 electrons.
Steps in Writing Electron Configurations [4:20]
To write electron configurations, first determine the atomic number of the element using the periodic table. The atomic number is typically found in the upper portion of the element's symbol on the periodic table. Next, use the electron configuration mnemonic device to arrange the energy sublevels in the correct order.
Principles Guiding Electron Configuration [5:56]
Three principles guide the writing of electron configurations. The Aufbau principle states that electrons first fill the lowest energy orbitals before occupying higher energy orbitals. Pauli's exclusion principle states that no more than two electrons can occupy an orbital, and these electrons must have opposite spins. Hund's rule states that for a set of orbitals with equal energy, electrons will occupy each orbital singly before any orbital is doubly occupied, and all single electrons will have parallel spins.
Hund's Rule and Magnetic Properties [8:55]
Hund's rule explains how electrons fill orbitals of equal energy. Electrons first occupy each orbital with one electron before pairing up. Elements with unpaired electrons are paramagnetic and are attracted to magnetic fields, while elements with all paired electrons are diamagnetic and are not attracted to magnetic fields.
Example: Phosphorus [11:47]
The video demonstrates writing the electron configuration for phosphorus, which has an atomic number of 15. Following the Aufbau principle, Pauli's exclusion principle, and Hund's rule, the electron configuration for phosphorus is 1s² 2s² 2p⁶ 3s² 3p³. The superscripts indicate the number of electrons in each sublevel, and their sum equals the atomic number of phosphorus.
Example: Oxygen [19:19]
The video provides another example, writing the electron configuration for oxygen, which has an atomic number of 8. The electron configuration for oxygen is 1s² 2s² 2p⁴.