TLDR;
This video provides a step-by-step guide on conducting antimicrobial testing using the Kirby-Bauer method. It covers preparing the media, creating bacterial suspensions, applying extracts and controls, incubating the samples, and measuring the inhibition zones to determine the antimicrobial effectiveness of substances like ginger and garlic.
- Preparing Muller Hinton agar plates aseptically.
- Creating bacterial suspensions of Escherichia coli and Staphylococcus aureus with a McFarland standard.
- Applying fresh spice extracts, positive (antibiotics), and negative (sterile distilled water) controls to the inoculated plates.
- Measuring and calculating the inhibition zones to determine antimicrobial effectiveness.
Introduction [0:00]
The video introduces a demonstration of antimicrobial testing using the Kirby-Bauer method. The presenter emphasizes the importance of maintaining a sterile environment throughout the experiment, starting with cleaning the work area with 70% alcohol.
Preparing the MH Medium and Petri Dishes [0:32]
The process begins with the preparation of Muller Hinton agar (MH) medium, which is already sterile. The narrator describes cleaning the outside of the medium bottle with 70% alcohol before aseptically pouring the medium into sterile petri dishes. The medium is poured to a depth of approximately 5mm, and the plates are left to solidify.
Making Fresh Spice Extract [1:40]
The video explains how to make fresh spice extracts from ginger and garlic. The ginger and garlic are peeled, washed with boiled water, and drained. Sterilized mortar and pestle are used to puree the garlic and ginger separately near a Bunsen burner. After pureeing, sterile water is added to each extract, homogenized, and transferred to small petri dishes, which are then labeled.
Creating a Suspension of Test Bacteria [3:47]
The process of creating a suspension of test bacteria, specifically Escherichia coli and Staphylococcus aureus, is detailed. These pathogenic bacteria have been incubated for approximately one day. The bacteria are dissolved in a physiological NaCl solution of 0.85% and compared visually to a 0.5 McFarland standard solution. The turbidity of the suspension is measured using a spectrophotometer at a wavelength of 600 nanometers, aiming for an absorbance of 0.1, equivalent to 1.5 * 10^8 CFU per milliliter.
Technique for Making Bacterial Suspension [5:34]
The narrator describes the technique of making a bacterial suspension using a 0.5 McFarland standard solution. A 0.85 percent NaCl solution is poured into the slanted agar, ensuring it's done over a Bunsen flame to maintain sterility. The solution is added to about half the height of the agar, and the tube is closed with a sterile stopper. The mixture is then stirred slowly to dissolve the bacteria without damaging the agar. The turbidity is visually compared to a tube containing a 0.5 McFarland standard solution. If the suspension is too cloudy, more NaCl solution is added; if it's too faded, more suspension from the slant agar is added until the turbidity matches the standard.
Checking Absorbance with Spectrophotometer [10:43]
The video demonstrates how to use a spectrophotometer to check the absorbance of the bacterial suspension. The wavelength is set to 600 nm, and the instrument is set to zero using a blank. The bacterial suspension is poured into a cuvette, and its absorbance is measured. The goal is to achieve an absorbance value of 0.1, which corresponds to a McFarland standard of 0.5. Adjustments are made by adding more concentrated suspension or diluting with NaCl until the desired absorbance is reached.
Antimicrobial Testing Stage [14:06]
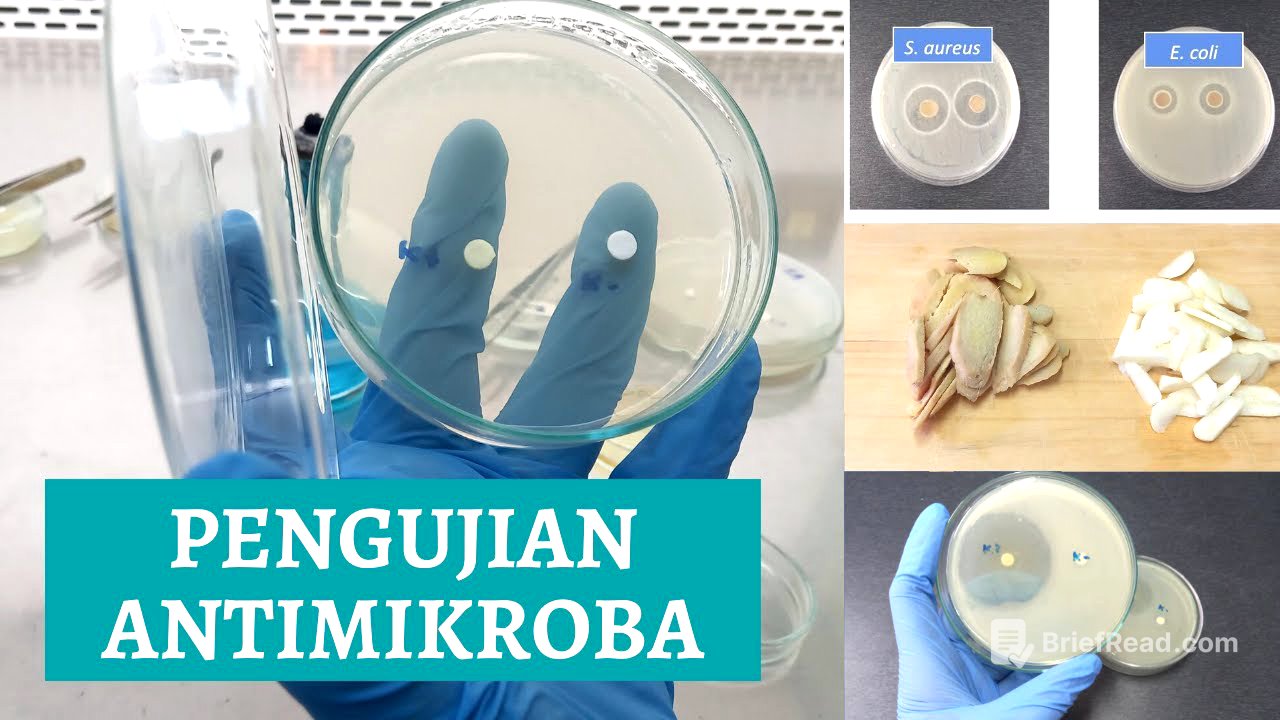
The prepared MH medium plates are labeled with codes for Escherichia coli (EC) and Staphylococcus aureus (SA). Each dish is streaked with the corresponding bacterial suspension using a sterile cotton swab in a zigzag pattern. The importance of using a new swab for different bacteria is emphasized to avoid contamination.
Applying Extracts and Controls [17:38]
In addition to the fresh ginger and garlic extracts, positive (antibiotics) and negative (sterile distilled water) controls are used. Sterile paper discs are soaked in each solution for about 10 minutes. The soaked discs are then placed on the inoculated agar plates, ensuring they are spaced apart and gently pressed to adhere to the surface.
Incubation [20:39]
After all the discs are placed, the petri dishes are wrapped with cling wrap and incubated at 37°C for 24 hours.
Observation of Results [21:30]
The results of the antimicrobial testing are observed, noting the presence of clear zones around the paper discs, which indicate inhibition of bacterial growth. The diameter of these clear zones is measured to quantify the effectiveness of the antimicrobial agents. The positive control (antibiotics) shows a clear zone, while the negative control (sterile distilled water) should not.
Measuring the Diameter of the Clear Zone [22:58]
The video explains how to measure the diameter of the clear zone using a ruler. For each clear zone, vertical and horizontal measurements are taken, including the diameter of the paper disc. The formula for calculating the inhibition zone is provided: (vertical diameter - disc diameter) + (horizontal diameter - disc diameter) / 2. An example calculation is given for ginger extract against S. aureus.
Calculating the Inhibition Zone [24:29]
After calculating the inhibition zone, the results are compared with a standard table to determine the antimicrobial effectiveness of the extracts. This comparison helps classify the substances as resistant, intermediate, or sensitive.