TLDR;
This video explains the first law of thermodynamics, which states that energy cannot be created or destroyed, but only transferred. It covers the concepts of internal energy, heat, and work, and how they relate to open, closed, and isolated systems. The video also discusses the sign conventions for heat (q) and work (w) in the context of chemistry, where the system's perspective is taken.
- Energy is conserved and can only be transferred.
- Internal energy changes are due to heat and work.
- Different types of systems (open, closed, isolated) have varying interactions with matter and energy.
- Sign conventions for heat and work depend on whether energy is absorbed/released by the system or done on/by the system.
Introduction to the First Law of Thermodynamics [0:01]
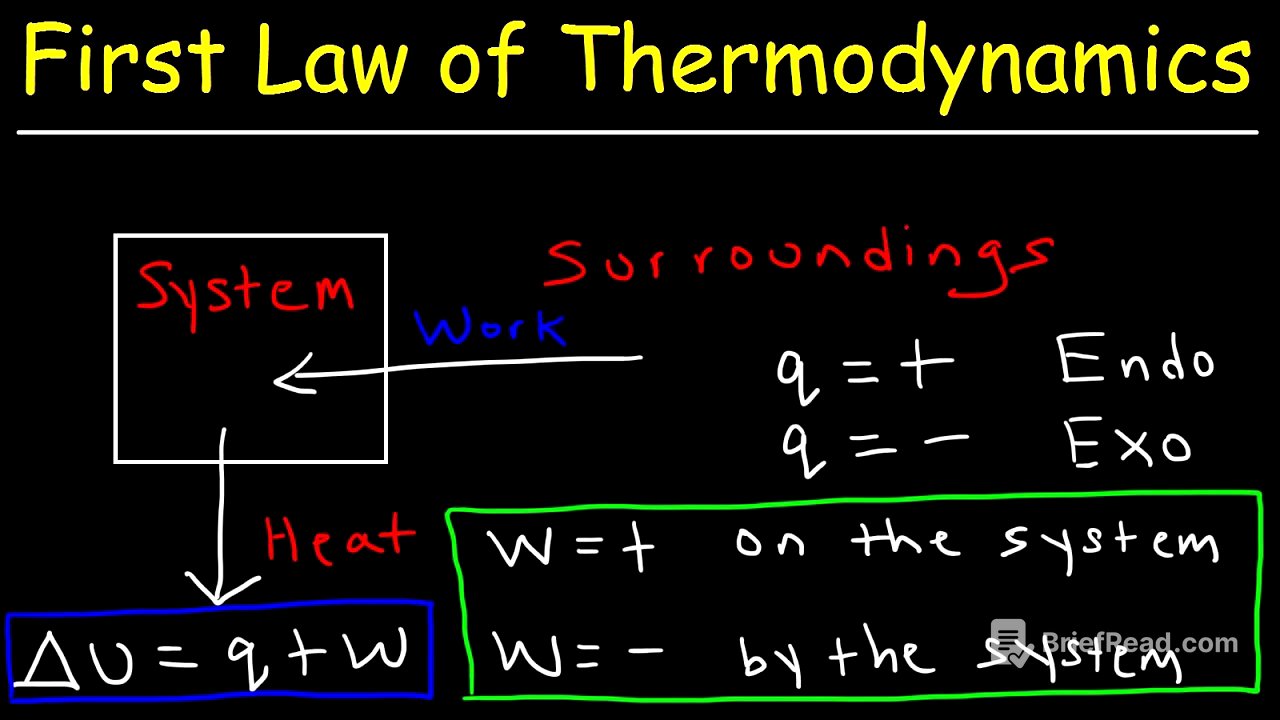
The first law of thermodynamics states that energy cannot be created or destroyed, but it can be transferred from one place to another. A system can exchange energy with its surroundings through heat and work. When heat flows into the system, the system gains internal energy, represented by the symbol U. The surroundings can also perform work on the system, increasing its internal energy. For example, if the surroundings perform 300 joules of work on a system, the system's internal energy increases by 300 joules, while the energy of the surroundings decreases by the same amount.
Illustrating Energy Transfer with an Example [2:08]
The principle of energy conservation can be illustrated with a simple example. If you sell a laptop for $500, your bank account increases by $500, while the buyer's bank account decreases by $500. The money doesn't appear from nowhere; it is transferred from one account to another. Similarly, if a system gains 300 joules of energy, the surroundings lose 300 joules of energy, and vice versa, demonstrating that energy is not created or destroyed but merely transferred.
Types of Thermodynamic Systems [3:47]
There are three types of systems: open, closed, and isolated. In an open system, both matter and energy can be transferred into and out of the system. In a closed system, energy can be transferred, but matter cannot. In an isolated system, neither energy nor matter can be transferred into or out of the system, meaning the mass and total energy within the system remain constant.
The Equation for Internal Energy Change [5:12]
The change in internal energy of a system is represented by the equation ΔU = q + w, where q is the heat energy that flows into or out of the system, and w is the work done. In chemistry, this equation is used from the system's point of view. However, in physics, the equation is often written as ΔU = q - w, because it takes the viewpoint of the surroundings.
Sign Conventions for Work (Chemistry vs. Physics) [5:43]
In chemistry, work (w) is negative when work is done by the system, as the system expends energy and its internal energy decreases. Conversely, work is positive when work is done on the system. In physics, the sign conventions are reversed because the viewpoint is that of the surroundings. When work is done by the system, the surroundings gain energy, so w is considered positive. When work is done on the system, the surroundings lose energy, and w is negative.
Analyzing Work Done By the System [7:51]
When work is done by the system, the internal energy of the system decreases. In chemistry, this is reflected by a negative value for w, indicating that the system is losing energy. In physics, the focus is on the surroundings, which gain energy when the system does work. Therefore, w is positive from the surroundings' perspective. The difference in sign conventions arises from whether the system or the surroundings is the focal point of the analysis.
Sign Conventions for Heat (q) [9:15]
Heat (q) is positive when the system absorbs heat energy, which is an endothermic process. Heat is negative when the system releases heat energy, which is an exothermic process. These sign conventions are consistent regardless of whether the analysis is from a chemistry or physics perspective.
Summary of Sign Conventions and Next Steps [10:28]
To summarize, in chemistry, w is positive when work is done on the system and negative when work is done by the system. It's important to be familiar with these sign conventions when using the equation ΔU = q + w. The video concludes by mentioning that future videos will provide practice problems on calculating the change in internal energy using heat and work.