TLDR;
This video explains acid-base titration, a method for determining the concentration of an acid or base in a solution. It covers the basics of titration curves, equivalence points, and indicators, and includes an example calculation to find the concentration of a sodium hydroxide solution.
- Titration involves determining the precise volume of acid or base needed to completely react with a solution.
- The equivalence point is when enough base has been added to neutralize the acid, often identified using an indicator.
- The pH at the equivalence point is 7 for strong acid-strong base titrations but varies when weak acids or bases are involved.
Introduction to Titration [0:00]
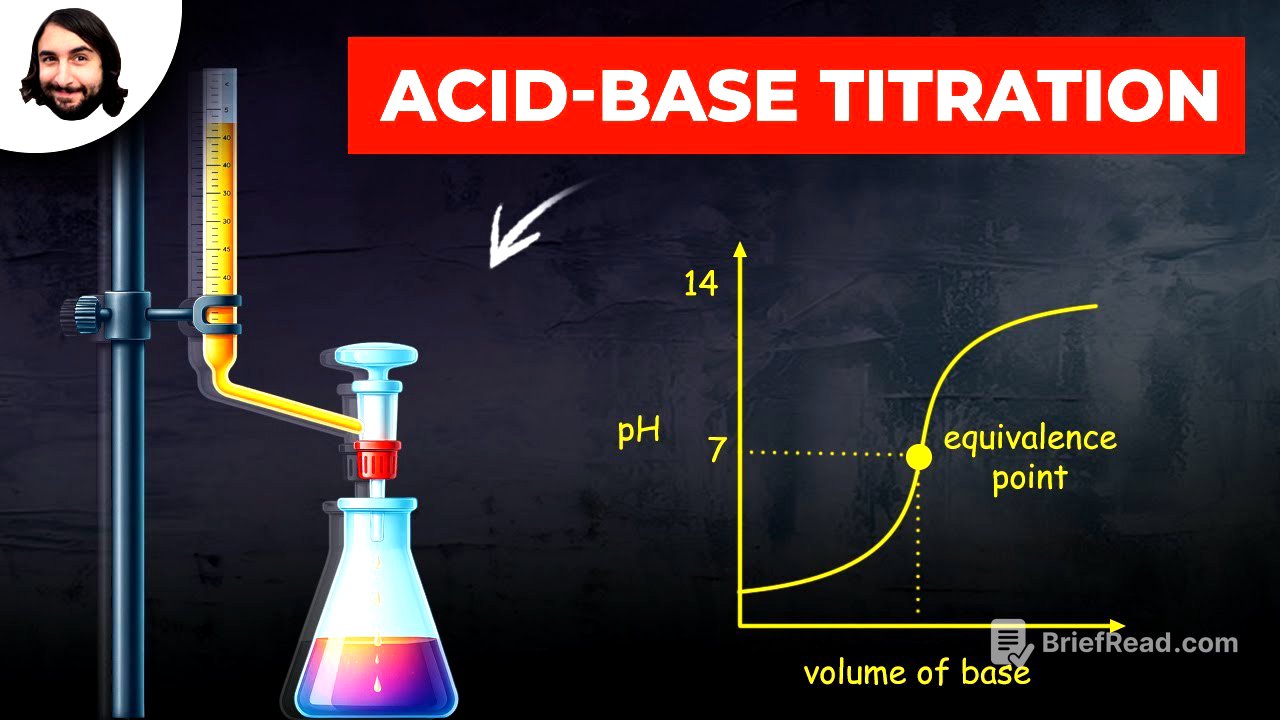
Acid-base titration is a technique used to determine the amount of acid or base present in a solution by finding the exact volume of acid or base required for a complete reaction. This process is similar to stoichiometry. A titration curve plots pH against the volume of base added to an acidic solution. Initially, the pH rises slowly, then increases sharply as it approaches the equivalence point.
Equivalence Point and Indicators [0:33]
The equivalence point is reached when precisely enough base has been added to neutralize the acid in the solution. Indicators, substances that change color at the equivalence point, are used to identify when this point is reached. For strong acid-strong base titrations, the equivalence point is at pH 7, but this value varies if weak acids or bases are involved.
Example Calculation [1:09]
To illustrate, consider determining the concentration of a sodium hydroxide solution. By reacting 3.0M sulfuric acid with 25 mL of the base, it's found that 11.6 mL of acid is needed to reach the equivalence point. Converting these volumes to liters and using the concentration of the acid along with the stoichiometric ratio allows for the calculation of the reactant's concentration.