TLDR;
This video explains the structure of atoms, what ions are, and how to interpret the nuclear symbol of an element. It covers the basic components of an atom, including protons, neutrons, and electrons, and their relative masses and charges. The video also explains how atoms can gain or lose electrons to become ions, and how the nuclear symbol provides information about the number of protons, neutrons, and electrons in an atom.
- Atoms are made up of protons, neutrons, and electrons.
- Protons and neutrons are located in the nucleus, while electrons orbit around the nucleus.
- Atoms can gain or lose electrons to become ions.
- The nuclear symbol of an element provides information about the number of protons, neutrons, and electrons in an atom.
The structure of an atom [0:29]
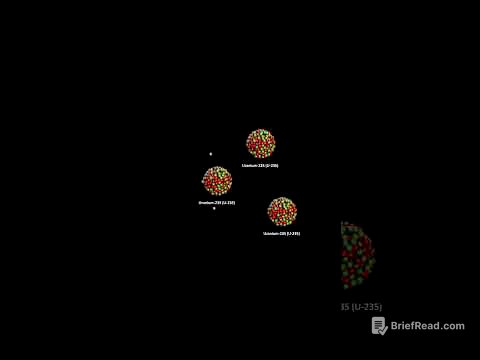
This chapter explains the basic structure of an atom. It describes the nucleus, which contains protons and neutrons, and the electrons that orbit around the nucleus in shells. The chapter also discusses the relative masses and charges of protons, neutrons, and electrons. Protons and neutrons have a relative mass of one, while electrons have a much smaller mass. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge.
What ions are [3:22]
This chapter explains what ions are and how they are formed. An ion is an atom that has gained or lost electrons, resulting in an overall charge. If an atom gains electrons, it becomes a negative ion, and if it loses electrons, it becomes a positive ion. The chapter uses examples to illustrate how the number of protons and electrons in an atom determines its charge.
What the nuclear symbol tells us [4:53]
This chapter explains how to interpret the nuclear symbol of an element. The nuclear symbol contains information about the element's atomic number, mass number, and elemental symbol. The atomic number represents the number of protons in an atom, which determines the element. The mass number represents the total number of protons and neutrons in an atom. The chapter explains how to calculate the number of neutrons in an atom using the mass number and atomic number. It also highlights that the atomic number also tells us the number of electrons in an atom.